Abstract
Introduction: MDS are a group of myeloid malignancies characterized by ineffective hematopoiesis, peripheral cytopenias, and 30% chance of transformation into AML. MDS pathogenesis involves, in some cases, a phenomenon of autoimmunity which can suppress normal hematopoiesis by direct cytotoxicity as well as by release of cytokines. Dysplasia-associated antigens released from dying cells might be responsible for evoking an adaptive immune response. NK cells appear activated, with high expression of perforin and granzyme, and mediate the cytotoxicity of bone marrow (BM) precursor cells in lower risk disease. Their activity is modulated by killer immunoglobulin-like receptors (KIR). Prior experience suggests that Tipifarnib, a farnesyl transferase inhibitor, may suppress abnormal NK cell function and autoimmunity that contributes to MDS. It is hypothesized that Tipifarnib could be especially of benefit in lower risk MDS patients where autoimmunity is known to play a role and immunosuppression is of clinical benefit in some cases.
KIR genes are polymorphic and two broad haplotypes exist: KIR-haplotype A mainly encode for inhibitory receptors and only for one activating (KIR2DS4), whereas group B haplotype encodes more for activating KIRs (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS5 und KIR3DS1). We hypothesized that KIR molecular profile could be correlated with response to Tipifarnib and molecular characteristics of patients with MDS. With this goal in mind, the key objective of the present study was to develop a methodology and a reportable range of KIR2DS2, KIR2DS5, KIR2DL2 and KIR2DL5 RNA expression and their ratios by RT-PCR in samples from low/INT-1 risk MDS patients.
Methods: We evaluated the KIR genotyping and gene expression from DNA and RNA samples, respectively, in BM mononuclear cells of 50 lower risk MDS patients followed at CUMC from 2010 to 2015. KIR typing was performed by conventional PCR using commercial Miltenyi KIR genotyping kit for all known 15 human KIR genes. Gene expression was accessed by qRT-PCR for two activating (2DS2, and 2DS5), two inhibitory (2DL2, and 2DL5) KIR genes, and five leukocyte markers: CD56, CD4, GZMM, CD8A, and CD8B . Correlation between clinical and molecular data of the patients was sought.
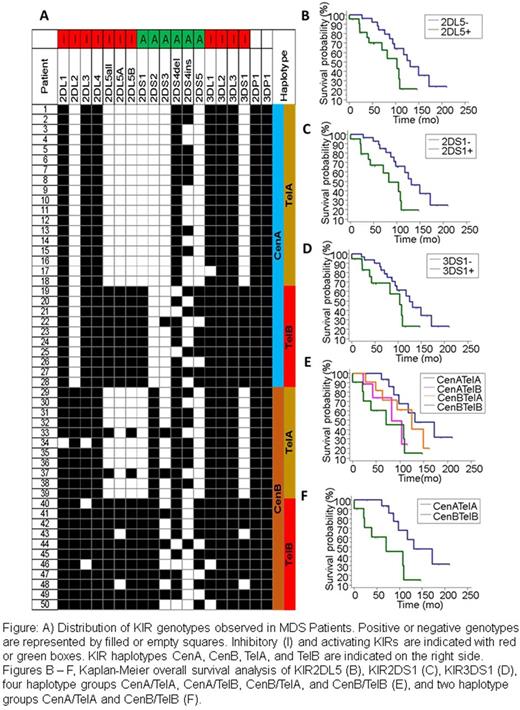
Results: Performance specifications (precision, limit of detection, linearity, and reportable range) were determined for all assays used in this study. All 50 MDS patients were positive for KIR2DL4, 3DL2, 3DL3, 2DP1 and 3DP1, whereas 2DS3 [7/55; 13%], 2DS5 [16/55; 29%], and 2DL5 [19/55; 35%] were less frequent (Figure 1). These results corroborate the existing literature showing similar distribution in the normal population. Patients with normal karyotype had higher frequency of 2DL5 [51% vs 33.3%], 2DS1 [46% vs 33.3%], 2DS3 [17% vs 6%], 2DS5 [37% vs 20%], and 3DS1 genes [43% vs 27%] than patients with abnormal cytogenetics, however none of these results was statistically significant. KIR2DS2 and 2DL2, and 2DS5 and 2DL5, co-occurred in the same patients, as already described in normal American population. All patients that presented KIR2DS2 [22/55; 44%], 2DL2 [22/55; 44%], 2DS5 [23/55; 46%], or 2DL5 [23/55; 46%] had their samples analyzed by qRT-PCR to the respective targets besides some leukocytes markers. Correlation between KIR copy number and MDS subtype, BM cellularity, WBC, RBC, Hg, and platelets was not observed.
Survival analysis showed poor survival for the patients carrying KIR2DL5, 2DS1, and 3DS1 genes [p<0.05] (Fig. B,C, & D). 42% (21/50) of the patients were positive for all three genes, showing the same ratio already identified in the normal population (36%). Additionally, survival was different between the four haplotypes groups CenA/TelA, CenA/TelB, CenB/TelA, and CenB/TelB (P=0.0628; Fig. E) but a subset analysis of CenA-TelA and CenB-TelB haplotypes showed a significantly different survival between two groups (P= 0.0145; Fig 1E). There was no correlation between gene expression data and survival.
Conclusions: We report on the methodology and its use to define a reportable range of KIR2DS2, KIR2DS5, KIR2DL2 and KIR2DL5 RNA expression and their ratios by RT-PCR in 50 lower risk MDS patients. Our findings suggest that KIR genes might be associated with some clinical features in patients, mainly, survival and cytogenetics profile. KIR expression will be correlated with clinical response to Tipifarnib as part of an ongoing clinical trial.
Coutinho: Kura Oncology: Consultancy. Ali: Onconova Therapeutics: Consultancy; Kura Oncology: Consultancy. Gualberto: Kura Oncology: Employment, Equity Ownership, Other: Chief Medical Officer, Patents & Royalties. Scholz: Kura Oncology: Employment, Equity Ownership, Patents & Royalties. Bracken: Kura Oncology: Employment. Jurcic: Forma Therapeutics: Research Funding; Celgene: Research Funding; Alexion Pharmaceuticals: Consultancy; Genentech: Research Funding; Incyte: Consultancy; Actinium Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Pharma, Inc: Research Funding; Daiichi-Sankyo: Research Funding; Kura Oncology: Research Funding; Merck: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Research Funding; Syros Pharmaceuticals: Research Funding; Amgen: Consultancy. Raza: Novartis: Speakers Bureau; Genoptix: Speakers Bureau; Celgene Inc.: Research Funding; Kura Oncology: Research Funding; Janssen R&D: Research Funding; Syros Pharmaceuticals: Research Funding; Onconova Therapeutics: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal